Warning
- Chain breaks within knot 31 (displayed as a gray area on the plot and as '-' on the sequence). The broken part of the chain has been replaced by a straight segment, which may affect what knot types are detected - be careful with interpreting results.

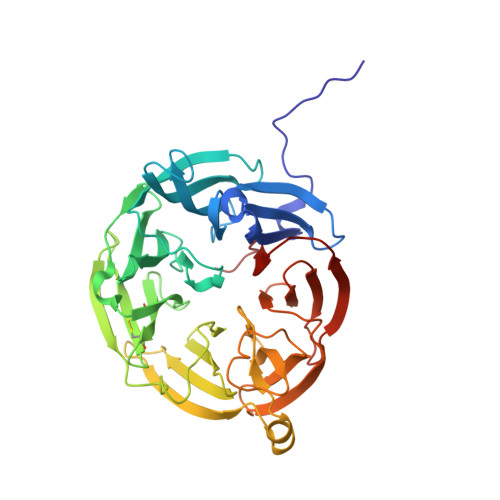
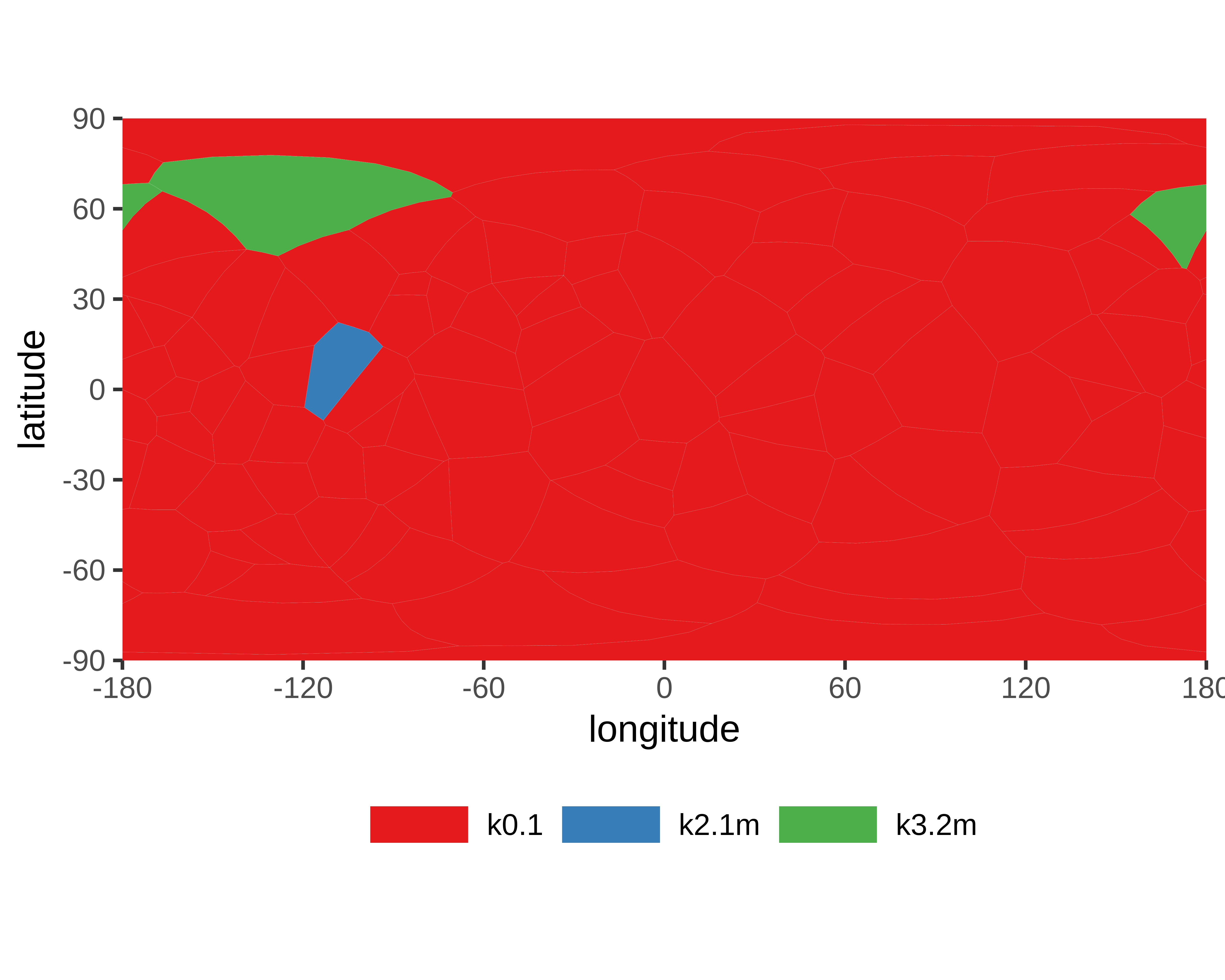
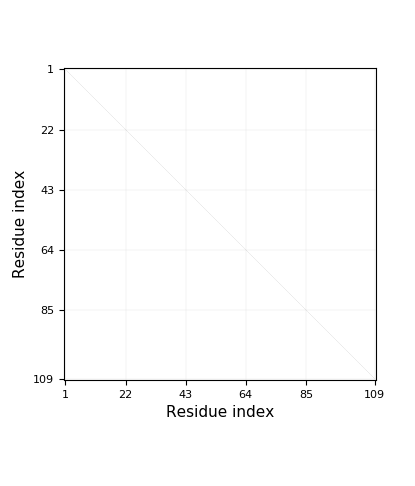

 Genus: 27
Genus: 27